Salicylic acid (SA) is a white, colorless crystalline powder widely used in anti-acne treatments. As a beta-hydroxy acid (BHA), it exhibits keratolytic, antimicrobial, and anti-inflammatory properties, while also helping to reduce sebaceous gland activity. Its exfoliating action makes it effective in treating conditions such as acne and seborrheic dermatitis, a skin disorder characterized by redness and scaling.
Clinical and experimental studies have shown that topical application of salicylic acid can help restore the proper organization of epidermal lipids, which strengthens the skin barrier, prevents microbial invasion, and supports normal cellular turnover. Additionally, the use of salicylic acid and its lipophilic derivatives has been associated with a reduction in fine lines, potentially helping to combat signs of photoaging. It is suitable for a wide variety of personal care and cosmetic products, including skin cleansers, shampoos, and products formulated for oily, acne-prone or blemished skin.
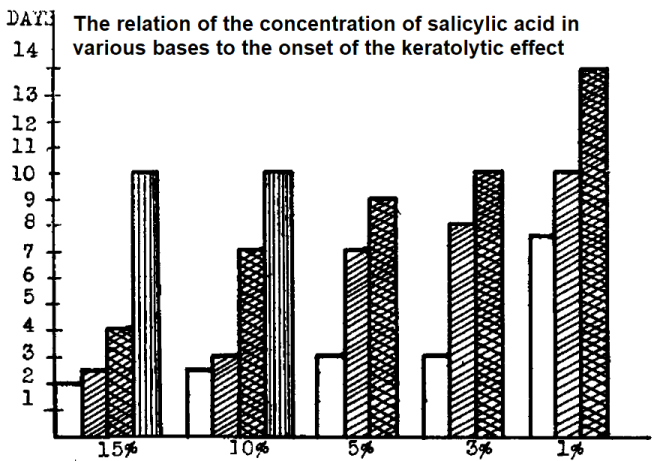
Salicylic acid is known for its activity at low pH due to its ionization potential. For daily leave-on products containing salicylic acid, it’s preferable to keep the pH below 4. The alkaline salt form, such as sodium salicylate, is considered less effective than the free acid. The keratolytic, or skin-peeling, effect of different salicylic acid bases (represented by the vertical bars) develops over time and varies with both the formulation and the salicylic acid concentration, as shown in the figure below [1]. Keep in mind that in the EU, when used for purposes other than preservation, salicylic acid is limited to 3% in rinse-off products and 2% in other cosmetic product types.
A word of caution: salicylic acid is lipophilic and, in its acidic form, has very limited solubility in water (about 2 g/L or 0.2% at 20 °C), which can make it challenging to work with at lower pH levels. Polysorbate 80 can help solubilize it in water when used at roughly a 1:7:92 ratio of salicylic acid, polysorbate 80, and water. Another option is to fully dissolve 2% salicylic acid by gradually incorporating it into a 50:50 betaine–water mixture. Oils are more effective solvents: salicylic acid dissolves up to about 2.2% in olive oil, around 12-16% in castor oil, and approximately 15–20% in octyldodecanol. Up to 30% pure salicylic acid can be dissolved in a specific non-hazardous glycol, representing the highest solubility attainable with conventional cosmetic solvents (excluding flammable options such as ethanol and isopropanol).
It’s best to avoid using a steel spatula when handling salicylic acid, as Fe³⁺ ions can form iron–salicylate complexes that may turn the mixture purple. Zinc also reacts with salicylic acid, forming salts that are inactive and generally regarded as inert.
[1] Strakosch E.A. Arch Derm Syphilol.









