Preservation by organic acids

Preservatives such as sodium benzoate and potassium sorbate are only effective in their acidic forms. When the pH is lowered, sodium benzoate converts to benzoic acid, and potassium sorbate becomes sorbic acid. These acidic forms are the active preservatives.
The effectiveness of these preservatives is closely tied to pKa — a term used to describe the strength of an acid. When the pH of a solution equals the pKa of a given acid, that acid is 50% dissociated. For instance, at this point, sodium benzoate exists as a 50/50 mix of benzoic acid and its salt form.
pKa is a measure of how strong an acid is: the lower the pKa, the stronger the acid. Strong acids, like hydrobromic acid (HBr, pKa = -9), dissociate more completely than weaker acids like sulfuric acid (H₂SO₄, pKa = -3) or acetic acid (pKa = 4.76).
Therefore, sodium benzoate and potassium sorbate are most effective as preservatives in low pH environments, where more of the compound exists in its active acid form. The image below from the Inolex brochure illustrates this concept clearly:
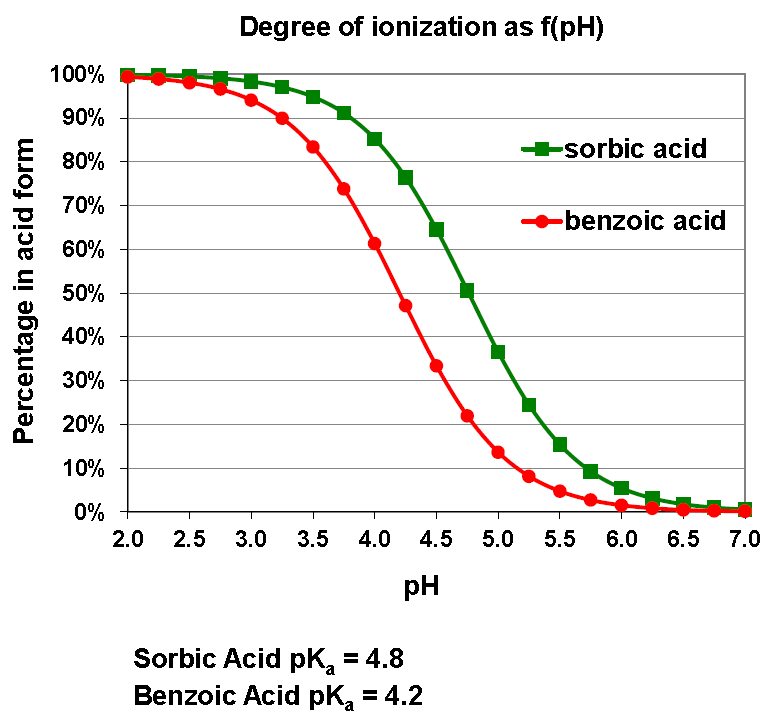
At pH 6, less than 10% of sodium benzoate and potassium sorbate exist in their active acid forms, resulting in minimal to no preservative efficacy.
This raises the question: Why do we use the sodium or potassium salts instead of the acids themselves?
The answer lies in formulation practicality — benzoic acid and sorbic acid have poor water solubility, making them difficult to incorporate directly. In contrast, their salt forms dissolve more easily in water, allowing for more efficient handling and uniform distribution in formulations.
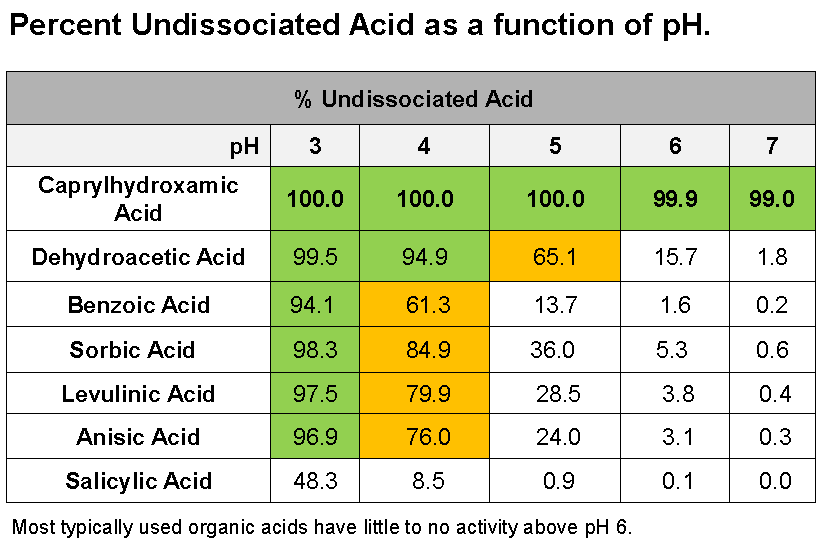
The table below (courtesy of Inolex) illustrates how the percentage of the active acid form of common acidic preservatives varies with pH.
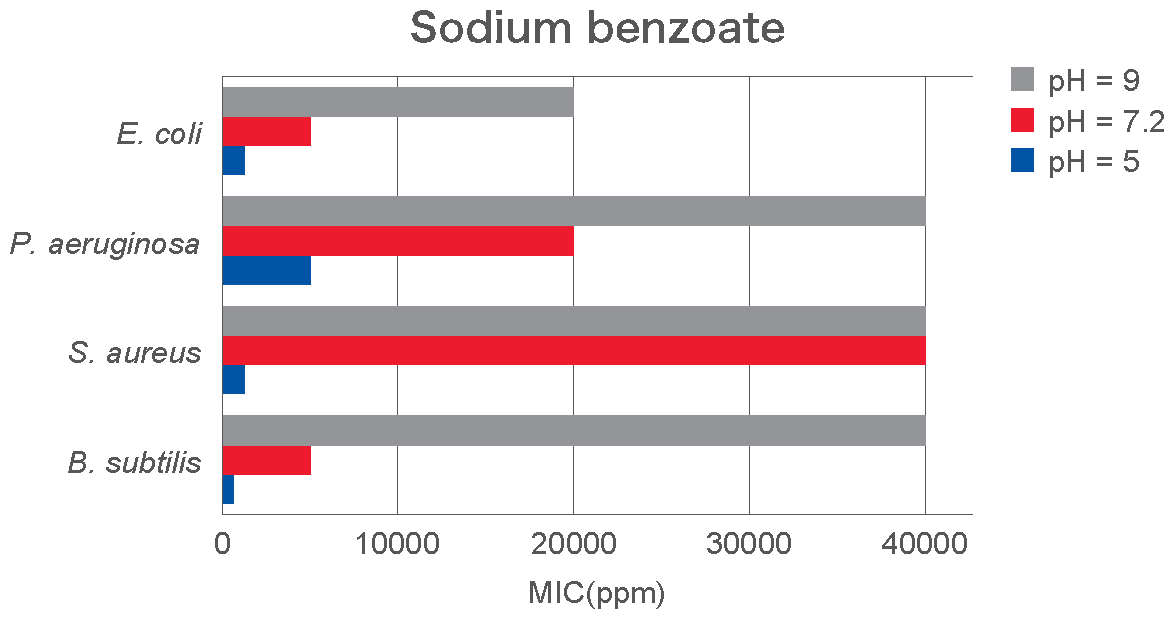
The graph below (courtesy of Adeka) provides another example of the Minimum Inhibitory Concentration (MIC) of sodium benzoate at acidic, neutral, and alkaline pH levels against various bacterial strains. It is evident that effectiveness increases significantly as the pH decreases, indicating the conversion of sodium benzoate to its active form, benzoic acid.