Irritative potential/mildness of surfactants

Surfactants are commonly classified according to their ionic charge, falling into four main categories:
- Anionic
- Cationic
- Amphoteric
- Non-ionic
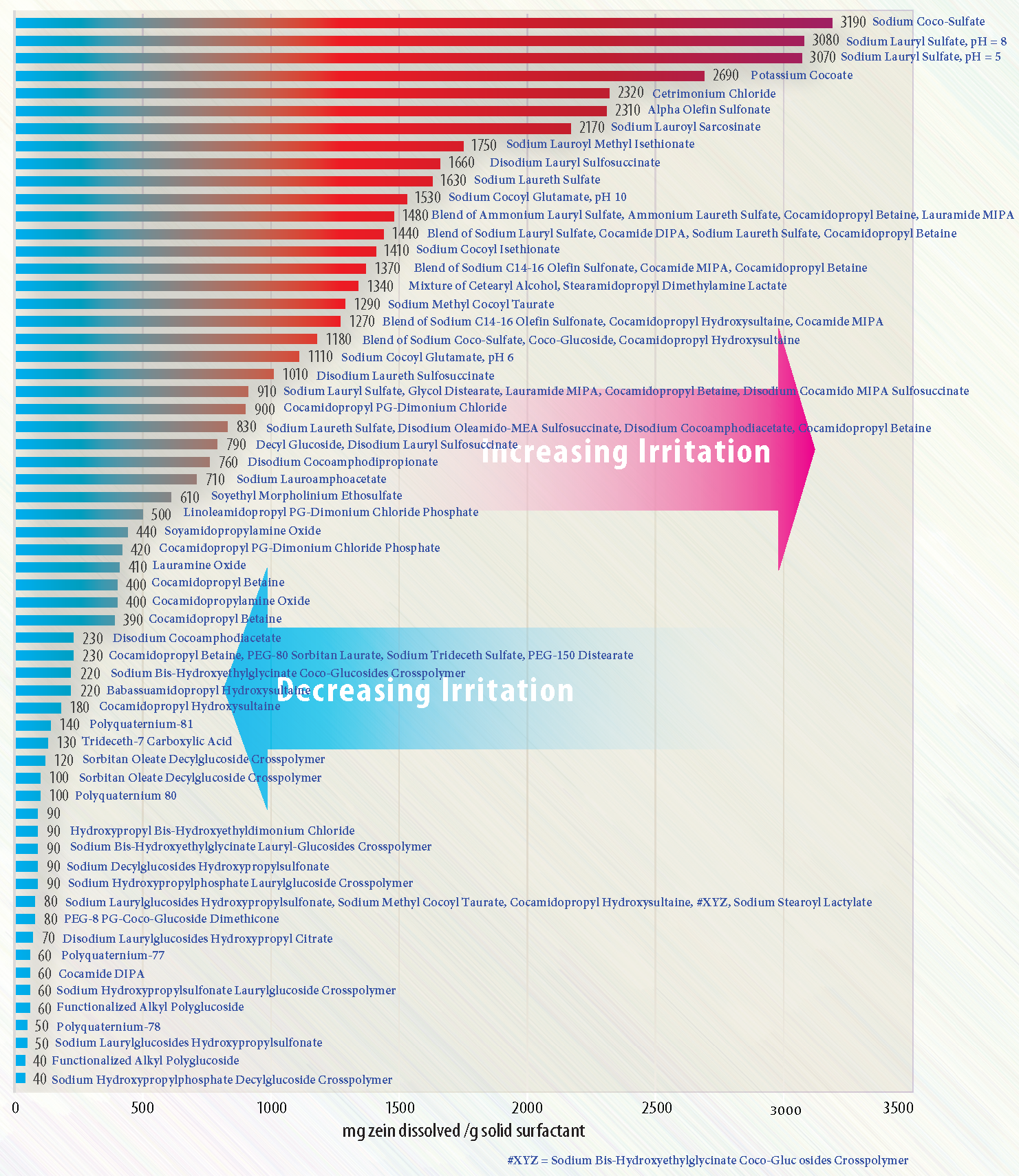
These classes not only differ in their physical and chemical properties but also in their degree of skin mildness. The potential for irritation in prototype cosmetic formulations is often assessed using the Zein test. This method utilizes zein, a corn-derived protein structurally similar to keratin found in the skin and hair. Zein is typically insoluble in water unless denatured.
In the test, a measured amount of zein protein is exposed to a solution containing surfactants from the cosmetic formulation. After the interaction, the denatured protein is separated. The Kjeldahl method is then employed to quantify the amount of nitrogen released, which serves as an indicator of protein denaturation. A higher nitrogen content suggests a greater degree of zein breakdown, and therefore, a higher irritation potential of the surfactant formulation. This test provides a useful in vitro approximation of how harsh a surfactant may be on the skin, helping formulators develop milder and more skin-friendly cosmetic products.
The same surfactant sourced from different manufacturers may exhibit varying Zein values due to slight differences in the percentage of active matter, as well as inconsistencies in testing procedures. Furthermore, mildness comparisons are often limited to only a few surfactants, making it challenging to assess larger sets in a standardized and meaningful way.
Colonial Chemical offers a mildness overview of their products based on a simplified, modified Zein test. While the method has certain limitations, it effectively reveals general trends in the irritation potential of various surfactants. The reported values for each product should not be interpreted as absolute measurements, but rather as comparative references.
In terms of skin irritation caused by pure surfactants, the general trend in decreasing order is: Anionic > Cationic > Amphoteric > Non-ionic. In practice, nearly all anionic surfactants, regardless of their specific chemistry, are too harsh to be used on their own. As a result, they are almost always blended with amphoteric or non-ionic surfactants, which significantly reduces their irritation potential.